3.2.4.1. The Concept and Method
Most measurements in chemistry and chemical analysis are performed on homogenous solutions, and except for kinetic assays, at a time when chemical equilibria are reached. This, batch approach, yields the data, which transformed alchemy to scientific discipline of chemistry.
For the reagent based chemical analysis in batch format, each sample (and standard solution) is assigned an individual container into which samples and reagents are metered, thoroughly mixed, and left to react until chemical equilibrium is reached. For a spectrophotometry, the reaction mixture is transferred into a cuvette for the absorbance measurement. Therefore measurement is performed on well defined (and known) composition of sample with reagent, at chemical equilibrium, and in stationary (not moving) solution. Consequently, batch based assays are more reproducible than flow based methods and are not affected by Schlieren Effect, viscosity or other matrix effects that manifest itself by difference between calibration obtained by flow based (kinetic) method with standards prepared in DI water, and with standards prepared in matrix of samples to be analyzed. Example such issue is determination of trace nutrients in sea water when salinity of standards must be matched with salinity of samples – a Herculean task when brackish waters are being surveyed.
To replicate conditions of conventional batch assay by means of flow programming, the following conditions have to be met:
- Sample and reagents must be precisely metered and homogenously mixed on the way to flow cell.
- Monitoring is performed at static conditions while the homogenous mixture of sample with reagents is held within the flow cell.
- The flow cell must be filled through its entire length with a reaction mixture of uniform composition (A). This is achieved:
- when the volume of the reaction mixture is equal to, or larger than, five times the volume of the conduit between the confluence point and the end of the optical path of the flow cell and
- the centroid of the reaction mixture is situated at the middle of the optical path of the flow cell.
- Absorbance values used to construct calibration graph are collected at a time when chemical reactions reached equilibria.
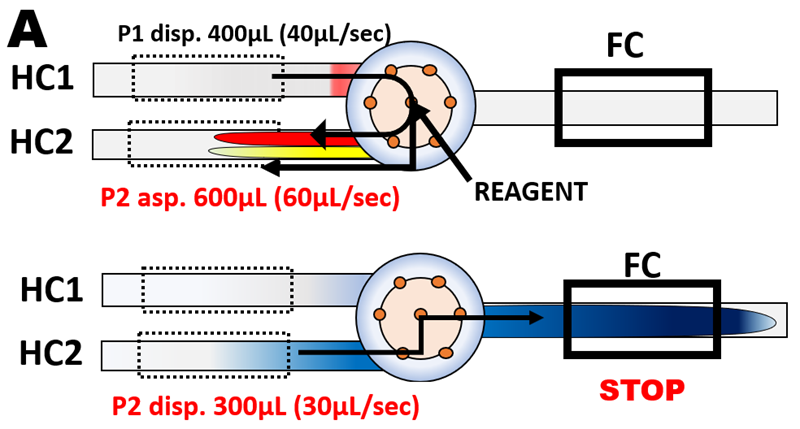
Details on how the condition 3. is met are given in Section 3.2.3. figures (A) to (F). Note that S+R volume of 600 µL and transfer volume TRv of 300 µL (A above) are selected because LOV module is furnished with 20 cm long flow cell (volume 100 µL). For shorter flow cell S+Rv and TRv can be reduced, for longer flow cells increased to meet condition 3. In this Chapter configuration of the last two steps of flow protocol shown above (A), was used in all experiments.
There are two areas where the use of batch mode FI is advantageous: for analysis of samples of high ionic content and as a tool for research and development of reagent based assays:
Development of Single Calibration Line method and its use for determination of trace nutrients in sea water (Hatta 2021), is the first analytical application of bFI. Section 3.2.4.2
Determination of molar absorptivities, dissociation constants, stability constants and of reaction rates, by bFI and comparison with data obtained by the conventional batch technique are summarized in Section 3.2.4.3.
Mariko Hatta, Jaromir (Jarda) Ruzicka, Christopher I. Measures, Madeline Davis “Programmable flow injection in batch mode: Determination of nutrients in sea water by using a single, salinity independent calibration line, obtained with standards prepared in distilled water “Talanta 232 (2021) 122354