Alzheimer´s Disease (AD) is a heterogeneous disease from pathophysiological aspects. The clinical form of this disease develops on the basis of various factor interactions. The deepening of degenerative neuronal damage (especially dendrites), their synaptic connections and cortico-subcortical atrophy induces the loss of memory and communication skills, psychosis, and after 3-7 years leads to death. The mechanism of neuronal death, or rather the initiation of the mechanism of death are not yet known.
The following basic pathophysiological processes assert in the development of AD:
1. Aß production
Aβ is a proteolytic product of Amyloid Precursor Protein (APP), it is highly neurotoxic. Its exact function has not been elucidated yet, it certainly contributes to the development of CNS response to stress or injury of central nervous system. APP undergoes proteolytic cleavage either amyloidogenic or non-amyloidogenic pathway.
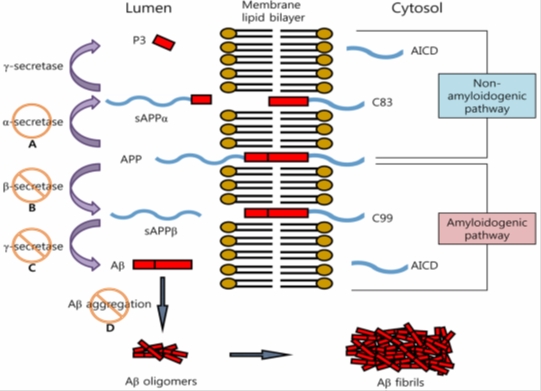
Fig. 1. Proteolytic processing of APP to β-amyloid (Aβ) [cited by: So-Young, P.: Arch. Pharm. Res. 33(10), 1589-1609 (2010)].
A – α-secretase elicitors, B – β-secretase inhibitors, C – γ-secretase inhibitors, D – aggregation inhibitors
α-secretase cleaves APP in 90% to short soluble fragments,
β-secretase BACE1 (β-site APP cleaving enzyme), cleaves APP at β-site, releases and changes soluble fragment (sAPPβ); membrane residue of protein is then cleaved by
γ-secretase (complex of PS1, PS2 etc.); proteins of 38-44 AA are released from the membrane rest of APP; the main of these fragments are:
Aβ1-40 – (occurrence in smaller proportion); these fragments tend to mis-furling, an increased accumulation in the wall of blood vessels, and initialize the development of amyloid angiopathy,
Aβ1-42 – (occurrence in major proportion); they cumulate mainly in neurons, initiate oxidative stress and accelerate the degeneration of neurons.
Accumulation of both Aß oligomers and polymers
- increases neurotoxicity above the activity of Aβ1-42 level,
- activates a cascade of enzymes responsible for the oxidative (or nitrogenous) stress,
- disrupts intracellular level of free Ca2+.
Potencial intervention
- α-secretase elicitors,
- BACE1 inhibitors,
- antioxidants and scavengers (ROS),
- inhibitors of neurotoxicity induced by Aβ,
- inhibitors of Aβ oligomers formation (resp. Aβ oligomers aggregation inhibition).
2. Senile plaque formation
Extracellular accumulation of Aβ surrounded by dystrophic neurites and microglia, or rather the high content of toxic Aβ oligomers (2-6 units) and insoluble β-sheet is according to amyloid hypothesis the main cause of AD pathology:
- decline of cognitive functions correlates with the level of Aβ,
- Aβ induces oxidative stress and inflammatory response, damages the neuronal synapses and dendrites.
Aβ1-42 is more amyloidogennic, it launches plaque formation; shortening and terminal glutamate cyclization to pyroglutamate on the N-terminus increases initiation of the capability for plaques formation 250times in comparison with the original Aβ1-40/42. This process is controlled enzymatically.
Aβ1-40 is deposited with Aβ1-42 into the plaque.
Potential intervention
- inhibitors of neuronal inflammation induced by Aβ,
- inhibitors of glutaminylcyclase,
- agonists of transcriptional factor PPARγ,
- compounds inhibiting oligomerization a polymerization of Aβ particles.
3. Tangles formation
Neurofibrillary tangles (intraneural accumulation of paired helical filaments) contain abnormally coiled up hyperphosphorylated τ-protein, modified neurofibrils and Aβ.
τ-Protein in neurons contributes to the incorporation of tubulin into microtubules and stabilizes them; τ-protein in tangles has higher Mr due to hyperphosphorylation, it drastically reduces the stability of microtubules. It also induces formation of Aβ and ROS:
- increased phosphorylation disrupts cytoskeleton and contributes to the decrease of nerve cells,
- microglia cells activated through inflammation in the periphery of amyloid plaques are released, loss of ATP occurs, and neurons solve this lack of energy by apoptosis.
GSK-3β, which starts degradation of intraneuronal τ-protein and phosphorylates microtubule-τ-protein in mammalian cells plays an important role.
Death of damaged neurons occurs mainly in cortical areas and subcortical structures of the limbic system. There is a reduced release of ACh from nerve endings that are related to decreased activity of the transport system, whose task is to accumulate ACh into nerve endings.
Potential intervention
- antioxidants and scavengers (ROS),
- GSK-3β inhibitors.
4. Interaction with rAGE (receptors for Advanced Glycation Endproducts)
Fibrils of Aβ interact with rAGE and scavenger receptors (RA) on the cell surface: increased levels of ROS are generated, and intracellular concentration of Ca2+ (due to depolarization of the cell membrane and activation of NMDA receptors) is also increased; both processes induce neuronal death (this process is aided by the lack of NGF or NGF receptors). Stimulation of rAGE in the microglia results in an increase of formation and release of NO, prostaglandins, excitotoxins, cytokines, TNF-α and fibroblast growth factor (B-FGF). Neuronal inflammation starts to develop.
Potential intervention
- rAGEs antagonists,
- compounds with anti-inflammatory activity,
- NGF elicitors.
5. Lower content of acetylcholine (ACh) in brain
During the development of AD exist in the brain tissues:
- a reduced production and release of ACh from presynaptic endings,
- a reduced level and activity of choline acetyltransferase,
- a reduced choline uptake into neurons and limited usability of acetyl CoA,
- a decrease of number of nicotine receptors (muscarinic ACh receptor number is relatively unchanged).
ACh is degraded in the synaptic cleft through acetylcholinesterase (AChE) (G4, G1), and also BuChE. Both enzymes are produced in an increased quantity in the area of Aβ plaque accumulation. Concentration of ACh is proportionally reduced with the degradation of nerve cell. In the large brain cortex and hippocampus levels of choline acetyltransferase decrease by up to 90%.
Also, decreasing concentrations of noradrenaline, somatotropin, neuropeptide Y, substance P, and others neuroregulators has been observed.
Potential intervention
- AChE and BuChE inhibitors (cognition enhancers),
- compounds improving brain metabolism (nootropics),
- prolylendopeptidase and prolyldipeptidase inhibitors.
6. Increased disturbance on NMDA receptors
During AD, the disturbance in transmission in glutamatergic system which is very important for learning and memory mechanisms, occurs. Irritation of NMDA receptors is associated with the opening of Ca2+ channels and with an increased Ca2+ entry into neurons. A chain of intraneuronal enzymes is activated. This process leads to destabilization of the internal homeostasis and apoptosis. The excessive release of glutamate and other excitatory acids will cause excessive irritation on NMDA receptors and production of transmission disturbance which make the right signals-potentials transfer impossible. These signals-potentials carry information of learning-content, and their damage plays very important role in the deterioration of AD.
Potential intervention
- inhibitors of NMDA receptors.