1. Nuclear receptors in xenobiotic and endobiotic metabolism regulation
Nuclear receptors (NRs) such as the Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and Farnesoid X receptor (FXR) are the most important endogenous ligand/xenobiotic-activated transcription factors responsible for both basal and inducible expression of genes involved in drug metabolism, glucose, lipid, cholesterol and bile acid homeostasis. However, selective human ligands and modulators are missing for specific descriptions of their regulatory functions in human hepatic cells. In addition, only little is known about the dynamics of cytochrome P450 enzyme regulation and posttranscriptional regulation of these NRs by miRNAs. The research aims to examine dynamics in target gene expression and regulation, and molecular mechanisms behind. The project is conducted in collaboration with Professor Stanislav Mičuda, PhD (Medical Faculty in Hradec Kralove, Charles University). In the development of novel cellular hepatocyte models including 3D spheroids of primary human hepatocytes, we collaborate with Magnus Ingelman-Sundberg (Karolinska Institutet).
In addition, we study if some environmental contaminants and industrial chemicals may affect hormonal and metabolism homeostasis due to the interaction with nuclear receptors. This research topic is studied in collaboration with the EDCMET H2020 consortium. Lipidomics analyses are performed together with Dr. K. Dohnalova (Czech Centre for Phenogenomics and 1st Faculty of Medicine, Charles University). Bile acid and cholesterol metabolomic analyses are performed in collaboration with Dr. M. Hroch (Faculty of Medicine in Hradec Kralove, Charles University) or Dr. Leniček and Prof. Vitek (1st Faculty of Medicine, Charles University).
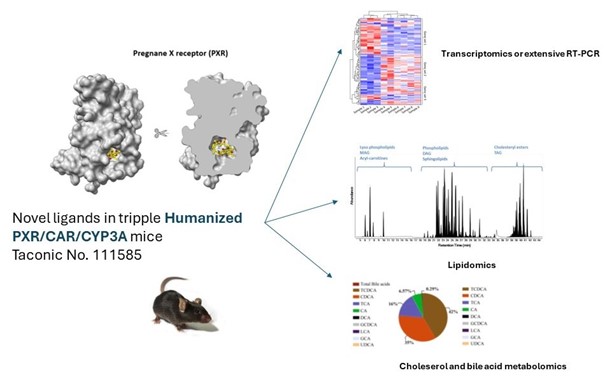
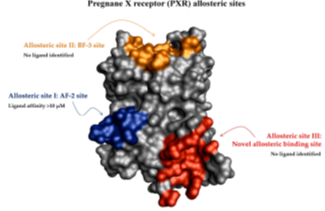
2. Novel ligands of nuclear receptors
In collaboration with Dr. Radim Nencka and Dr. Eva Kudova (Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Prague) and Associate Professor Jaroslav Roh (Faculty of Pharmacy, Charles University), we are developing and testing novel ligands for several nuclear receptors. In collaboration with colleagues from our faculty, we are also involved in the development of novel antituberculosis drugs (Dr. J. Roh´s group). Candidate compounds are tested in collaboration with Dr. Karel Chalupský (Czech Centre for Phenogenomics) and Prof. S. Micuda and Dr. M. Hroch (Faculty of Medical, Charles Universty). 3D modeling and in silico prediction are performed in collaboration with Dr. Thales Kronenberger (University of Tübingen and University of Eastern Finland). Lipidomics analyses are performed in collaboration with Dr. K. Dohnalova (Czech Centre for Phenogenomics and 1st Faculty of Medicine, Charles University). Bile acid and cholesterol metabolomic analyses are performed in collaboration with Dr. M. Hroch (Faculty of Medicine in Hradec Kralove, Charles University) and Dr. Leniček and Prof. Vitek (1st Faculty of Medicine, Charles University).
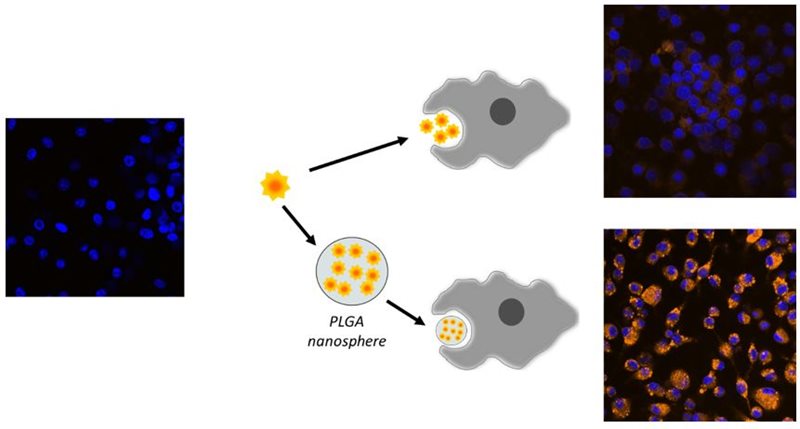
3. Novel formulation for the delivery of nuclear receptor ligands
We are developing novel nanoformulation for the delivery of nuclear receptors ligands into cells of immune system. APIs of interest are small molecules as well as nucleic acids (such as siRNA). We focus on PLGA nanoparticles and lipid nanoformulations (liposomes, solid-lipid nanoparticles, SNEDDS) based on cationic lipids. We collaborate with Professor Anette Mullertz (University of Copenhagen) and with Professor Daniel Scherman (Centre National de la Recherche Scientifique (CNRS).
4. Radiopharmacy
The work is concentrated on potential radiopharmaceuticals from two groups: radiolabeled receptor-specific peptides and radiolabeled monoclonal antibodies. The research includes some pharmacodynamic aspects of drug-organism interactions such as evaluation of radiolabeled ligand binding to selected targeted receptors (megalin, LRP2) in the kidney. Concomitantly, we investigate selected aspects of pharmacokinetics of the tested compounds. The studies are aimed at absorption, biodistribution, renal and liver elimination but we also carry out experimental studies on total pharmacokinetics in vivo.
Last update: 22/04/2024