1. The way from the synthesis of candidate antimicrobial compound to final steps in preclinical research studies
Antimicrobial therapy is one of the pillars of modern medicine. Unfortunately, microbial resistance's natural phenomenon is rapidly increasing, and infectious microorganisms' resistance to therapeutics is a severe, growing threat. Based on this fact, searching for new chemical compounds with antimicrobial activity seems to be the logical, required, and well-founded step.
In our microbiological laboratories, meeting the condition for BSL-2, for evaluation and characterization of antimicrobial action of candidate test compounds, different methodical approaches and methods are employed.
1.1 Testing of antimicrobial activity – basic and advanced screening of antibacterial and antifungal activity
For the basic screening and evaluation of the antibacterial and antifungal activities, we employ methodical approaches and testing methods (macro/microdilution broth methods, Fig. 1; disc diffusion methods, Fig. 2) based on internationally accepted standard methods CLSI (Clinical&Laboratory Standard Institute) and EUCAST (The European Committee on Antimicrobial Susceptibility Testing), and reference, collection bacterial and fungal strains (Table 1).
 Fig. 1 Microdilution broth method
Fig. 1 Microdilution broth method
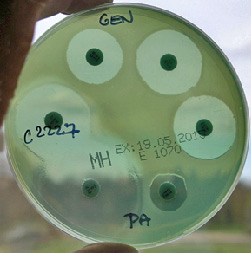 Fig. 2 Disc diffusion method
Fig. 2 Disc diffusion method
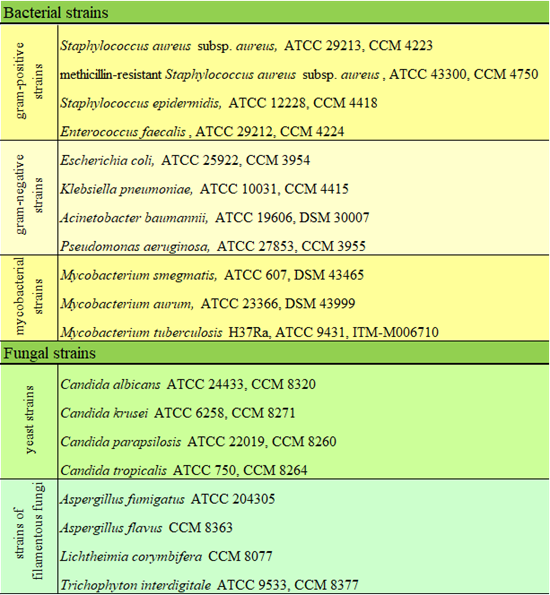 Table 1: List of microbial reference strains used for basic screening of anti-infective activity
Table 1: List of microbial reference strains used for basic screening of anti-infective activity
As part of the confirmation of previous results, extended in vitro testing of antimicrobial activity against the medically significant clinical isolate strains is performed. All clinical isolate strains are kindly provided by our co-workers, Dr. Voxová and Dr. Vejsová, from University Hospital Hradec Králové, Department of Clinical Microbiology. Strains are taxonomically classified by biochemical tests and MALDI-TOF instrumentation. The susceptibility/resistance profile of strains is determined by the disc diffusion method according to EUCAST recommendation.
In our department, we also deal with many other aspects and parameters that should be evaluated in preclinical research studies of anti-infective compounds.
1.2 Determination of static vs. cidal activity and microbial killing kinetic profiles in vitro
For the characterization of test compounds in preclinical studies, the recognition between static vs. cidal effect is evaluated (Fig. 3). In compounds with cidal effect in vitro, the time-kill kinetic profiles against microbial strains are evaluated, as well (Fig. 4).
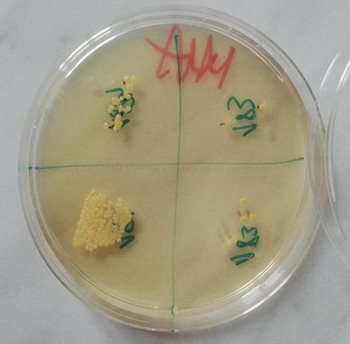 Fig. 3 Spot plate technique for evaluation of bactericidal vs. bacteriostatic action
Fig. 3 Spot plate technique for evaluation of bactericidal vs. bacteriostatic action
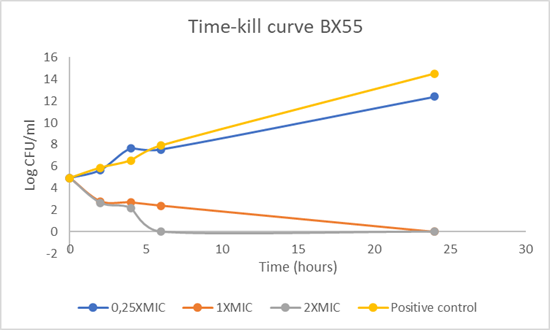 Fig. 4 Kinetic profile of bactericidal action of test compound designated BX55
Fig. 4 Kinetic profile of bactericidal action of test compound designated BX55
1.3 Insight into mechanism of action
For evaluation of the target microbial structures affected by test compound, the macromolecular biosynthesis assays and membrane depolarization assay are employed.
1.4 Toward to combination therapy - “partner” drug searching
The synergic, additive, indifferent or antagonistic effect in vitro of two or more drugs/test compounds in combination is revealed by checkerboard studies (Fig. 5). The FIC (Fractional Inhibitory Concentration) index value is calculated to evaluate of the impact of drug combinations (Fig. 6).
 Fig. 5 Checkerboard assay. Combination of different concentrations of test compound (ChO-W) and ciprofloxacin (CIP). Metabolic indicator Alamar Blue was employed for evaluation of bacterial viability.
Fig. 5 Checkerboard assay. Combination of different concentrations of test compound (ChO-W) and ciprofloxacin (CIP). Metabolic indicator Alamar Blue was employed for evaluation of bacterial viability.
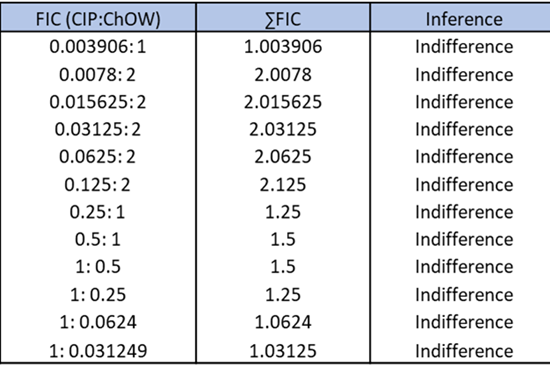 Fig. 6 Calculation of total fractional inhibitory concentration index (FIC).
Fig. 6 Calculation of total fractional inhibitory concentration index (FIC).
1.5 Screening of anti-biofilm activity
The MBIC (minimum biofilm inhibitory concentration), MBEC (minimum biofilm eradication concentration), and BPC (biofilm prevention concentration) are determined by microtitre plate biofilm assay, or methodical approach analogous to the Calgary Biofilm device is employed for evaluation of parameters mentioned above.
1.6 Evaluation of in vivo toxicity of test compounds in animal model, Galleria mellonella
In promising compounds with antimicrobial activity, the in vitro cytotoxicity is evaluated (cooperation with Assoc. Prof. František Trejtnar, Ph.D., Department of Pharmacology and Toxicology, Faculty of Pharmacy in Hradec Králové, Charles University). If two criteria are met (promising antimicrobial activity and non-cytotoxicity/low cytotoxicity in vitro), in vivo toxicity using the model, Galleria mellonella, is evaluated (Fig. 7, 8).
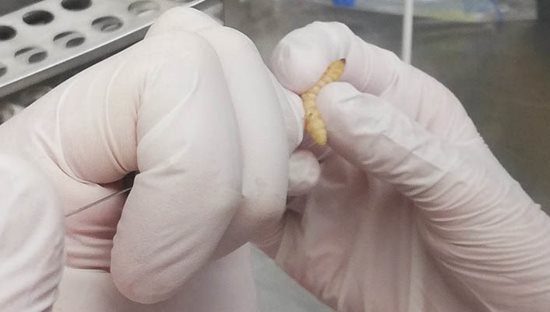 Fig. 7 Administration of test compound into larva of Galleria mellonella
Fig. 7 Administration of test compound into larva of Galleria mellonella
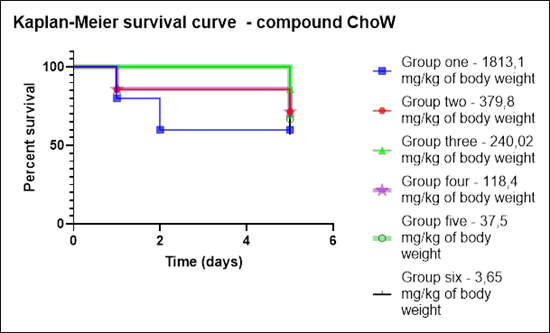 Fig. 8 Evaluation of Kaplan-Meier survival curve for test compound designated ChoW
Fig. 8 Evaluation of Kaplan-Meier survival curve for test compound designated ChoW
1.7 Evaluation of in vivo antimicrobial action of test compounds – modeling of treatment procedures in vivo
In our department, methodical approaches for the evaluation of in vivo activity of test compounds are introduced, as well.
2. Study of the experimental settings leading to robust and reproducible bacterial and fungal biofilm formation in vitro
The basic step leading to obtaining reproducible and valid results from in vitro anti-biofilm activity evaluation consists in the formation of sufficiently representative biofilms with characteristic attributes presented in biofilms produced in vivo. Based on this assumption, factors affecting the formation of yeast (Fig. 9) and staphylococcal biofilms (Fig. 10) in vitro are the aim of our interest.
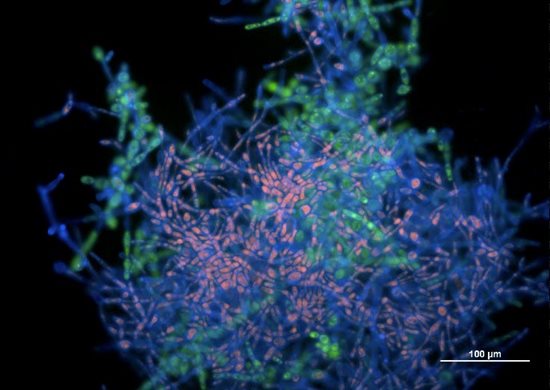 Fig. 9 Picture from epifluorescence microscopy. Biofilm biomass of Candida tropicalis
Fig. 9 Picture from epifluorescence microscopy. Biofilm biomass of Candida tropicalis
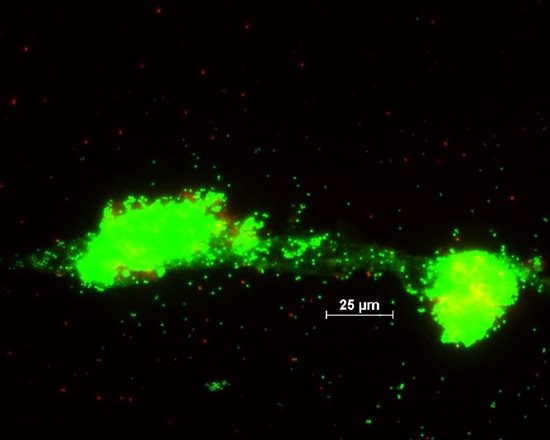 Fig. 10 Picture from epifluorescence microscopy. Biofilm biomass of Staphylococcus aureus
Fig. 10 Picture from epifluorescence microscopy. Biofilm biomass of Staphylococcus aureus
3. Study of Candida albicans host-pathogen interaction
Candida albicans (C. albicans) is a polymorphic fungus causing infections that range from superficial infections of the skin to life-threatening systemic infections. Although several virulence factors have been recently revealed, further investigations on a molecular basis for understanding the pathogenicity mechanism of C. albicans are required. Clarification of pathogenic mechanisms on the molecular basis offers the application of a new therapeutic strategy of C. albicans infections, utilizing the strategy of virulence factors targeting.
Our research was focused on isolation and identification of proteins transported from C. albicans (reference and clinical isolate strains) via extracellular vesicles (Fig. 11) and subsequent categorization by bioinformatic analysis (Fig. 12).
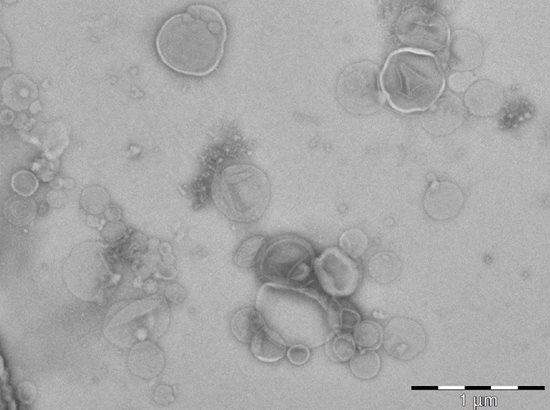 Fig. 11 Picture from transmission electron microscopy (coopeartion with Dr. O. Benada, Institute of Microbiology of the Czech Academy of Sciences, v.v.i, Czech Republic). Extracellular vesicles of Candida albicans present in culture filtrates.
Fig. 11 Picture from transmission electron microscopy (coopeartion with Dr. O. Benada, Institute of Microbiology of the Czech Academy of Sciences, v.v.i, Czech Republic). Extracellular vesicles of Candida albicans present in culture filtrates.
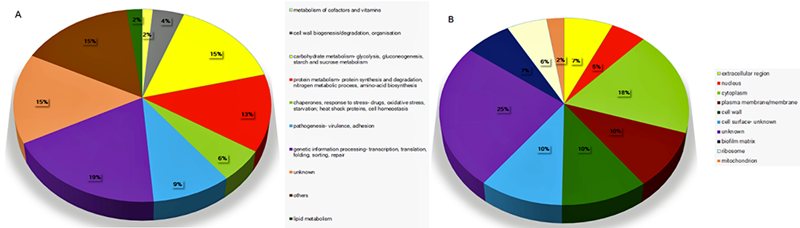 Fig. 12 A pie chart of the protein groups found in EVs isolated after the cultivation of the C. albicans strain ATCC 90028, in a nutrient-limited medium. The identified proteins from EVs were sub-divided into ten groups according to their proposed function (A) and sub-cellular localization (B).
Fig. 12 A pie chart of the protein groups found in EVs isolated after the cultivation of the C. albicans strain ATCC 90028, in a nutrient-limited medium. The identified proteins from EVs were sub-divided into ten groups according to their proposed function (A) and sub-cellular localization (B).