The functional unit of the placenta, i.e. the polarized cells of trophoblast, expresses a number of transport proteins localized either in the apical brush border (maternal-facing) membrane or basolateral (fetal-facing) membrane. Several of these transporters have been described to control the transplacental disposition of many drugs and play a crucial role in fetal protection against maternal toxins.
ABC transporters in the placenta
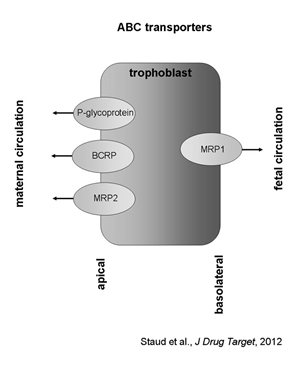 The ATP-binding cassette (ABC) superfamily comprises 48 members divided into seven subfamilies of membrane proteins that transport a wide variety of substrates (Dean and Annilo, 2005). In terms of drug transport, members of three main subfamilies are of particular importance, i.e. P-glycoprotein (MDR1, ABCB1), breast cancer resistance protein (BCRP, ABCG2) and multidrug resistance-associated proteins (MRPs, ABCCs). These drug transporters are primarily known for their role in multidrug resistance in cancer, however, they have also been confirmed to play a significant role in the pharmacokinetics, affecting drug absorption, elimination, and distribution across blood-tissue barriers, including placenta (Bodo et al., 2003; Leslie et al., 2005; Fromm and Kim, 2011). In the placenta, ABC transporters actively pump their substrates out of the trophoblast cells into the maternal (P-glycoprotein, BCRP, MRP2) or fetal (MRP1) circulation. To date, ABC drug efflux transporters localized to the apical membrane of the trophoblast are considered to be the main active components of the placental barrier.
The ATP-binding cassette (ABC) superfamily comprises 48 members divided into seven subfamilies of membrane proteins that transport a wide variety of substrates (Dean and Annilo, 2005). In terms of drug transport, members of three main subfamilies are of particular importance, i.e. P-glycoprotein (MDR1, ABCB1), breast cancer resistance protein (BCRP, ABCG2) and multidrug resistance-associated proteins (MRPs, ABCCs). These drug transporters are primarily known for their role in multidrug resistance in cancer, however, they have also been confirmed to play a significant role in the pharmacokinetics, affecting drug absorption, elimination, and distribution across blood-tissue barriers, including placenta (Bodo et al., 2003; Leslie et al., 2005; Fromm and Kim, 2011). In the placenta, ABC transporters actively pump their substrates out of the trophoblast cells into the maternal (P-glycoprotein, BCRP, MRP2) or fetal (MRP1) circulation. To date, ABC drug efflux transporters localized to the apical membrane of the trophoblast are considered to be the main active components of the placental barrier.
SLC transporters in the placenta
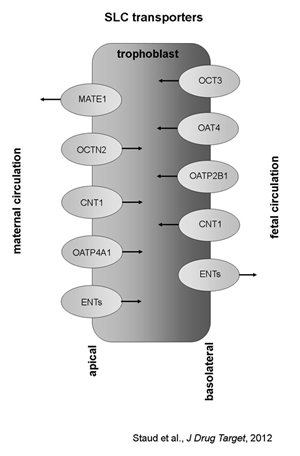 While the expression, localization and function of ABC drug efflux transporters in the placenta have been relatively well described using a variety of experimental approaches in several mammal species, much less attention has been paid to the role of the solute carrier (SLC) family of transporters in the placenta. This transporter superfamily is by far the largest family of transporters, consisting of over 300 members that have been described in many tissues throughout the body. Some of the members are relatively substrate-specific, mediating transport of endogenous compounds such as sugars, amino acids or nucleosides; some other members, on the contrary, show wide substrate specificity, recognizing broad spectrum of molecular structures and dimensions. These are called “polyspecific” transporters and play a major role in drug elimination, especially in excretory organs, typically kidney and liver (Koepsell and Endou, 2004).
While the expression, localization and function of ABC drug efflux transporters in the placenta have been relatively well described using a variety of experimental approaches in several mammal species, much less attention has been paid to the role of the solute carrier (SLC) family of transporters in the placenta. This transporter superfamily is by far the largest family of transporters, consisting of over 300 members that have been described in many tissues throughout the body. Some of the members are relatively substrate-specific, mediating transport of endogenous compounds such as sugars, amino acids or nucleosides; some other members, on the contrary, show wide substrate specificity, recognizing broad spectrum of molecular structures and dimensions. These are called “polyspecific” transporters and play a major role in drug elimination, especially in excretory organs, typically kidney and liver (Koepsell and Endou, 2004).
In general, SLC transporters in the placenta mostly facilitate energy-independent uptake of hydrophilic or charged molecules by the trophoblast cells. Once in the trophoblast, these substrates might be either utilized for placenta´s own need (such as de novo synthesis of placental hormones) or pumped out of the cell by another, SLC or ABC transporter. The following SLC transporters have been identified in the placenta: organic cation transporters (OCTs), organic anion transporters(OATs), carnitine transporters (OCTNs), nucleoside transporters (CNTs and ENTs), organic anion transporting polypeptides (OATPs) and the recently discovered multidrug and toxin extrusion proteins (MATE).