3.2.5.6. Selectivity of the simplified method for silicate determination in sea water is evaluated by autocalibration
J. Ruzicka, M. Davis, Ch. Measures M. Hatta, © August 2022
The aim of this section is to introduce autocalibration as an efficient tool for evaluation of selectivity of determination and to present a simplified version of the molybdenum blue method based on the use of blended oxalic/ascorbic acids reagent.
Selectivity of a determination is usually described as ratio of concentrations of interfering to target species at which influence of the former is observed on finding of the latter. While reassuring, this estimate does not provide an information on the range of concentrations and degree of interference beyond described interference ratio. Use of ratio of calibration lines, obtained by serial dilution would be more informative but it is labor and time consuming. However, autocalibration by single standard solution (Section 3.2.5.2.) makes evaluation and measurement of selectivity practical.
Silica and phosphate are essential nutrients and their distribution in oceans, fresh water, soils, and plants is most frequently measured by spectrophotometry based on formation molybdenum blue method (MoB). Discovered 200 years ago MoB method has been described in several thousands of papers in dozens of modifications, and its underlying chemistry is so complex that authors who recently published an excellent, comprehensive review [1] decided to entitle their paper “Molybdenum blue reaction for the determination of orthophosphate revisited: Opening the black box.”
Briefly, seen from a view of a practicing analyst, there are three species to be identified and measured: phosphomolybdenum blue (PMoB with bands at 880nm and 740nm), silicomolybdenum blue (SiMoB with band at 810nm) and molybdenum blue (MoB with broad absorbance in 400 to 900nm range). The first two species are formed by reduction in acid medium from soluble phosphate and silicate in presence of molybdic acid. In a strongly acid solution (pH 0 to 1) only phosphomolybdate (PMo) is formed in presence of Si, thus allowing selective determination of phosphate to be performed. With decreasing acidity (pH 2 to 3) SiMo becomes formed as well, and since PMoB and SiMoB absorption spectra are broad and overlap, phosphate interferes in determination of silica. To avoid this interference the most widely used method is based on masking with oxalic acid which both binds excessive Mo into nonreducible complex and decomposes PMo thus preventing formation of interfering PMoB. [1 to 4 and 6 ]
Therefore, methods for determination of silica are based on three steps: formation of SiMo+PMo, decomposition of PMo, and reduction of remaining SiMo into SiMoB measured at 810nm.
When automated in Flow Injection format [2] the three reagents are used in sequence (A). Thus sample (S) is injected into DI water carrier, merged with Mo reagent that forms SiMo+PMo in coil #1. Next, stream of oxalic acid is merged to decompose PMo and to deactivate Mo reagent by complex formation in coil #2. Finally, ascorbic acid reduces SiMo into SiMoB in coil #3. Blending of reagents to simplify the flow protocol is not deemed feasible because oxalic acid will deactivate Mo reagent, while mixture of ascorbic acid with Mo reagent is not stable.
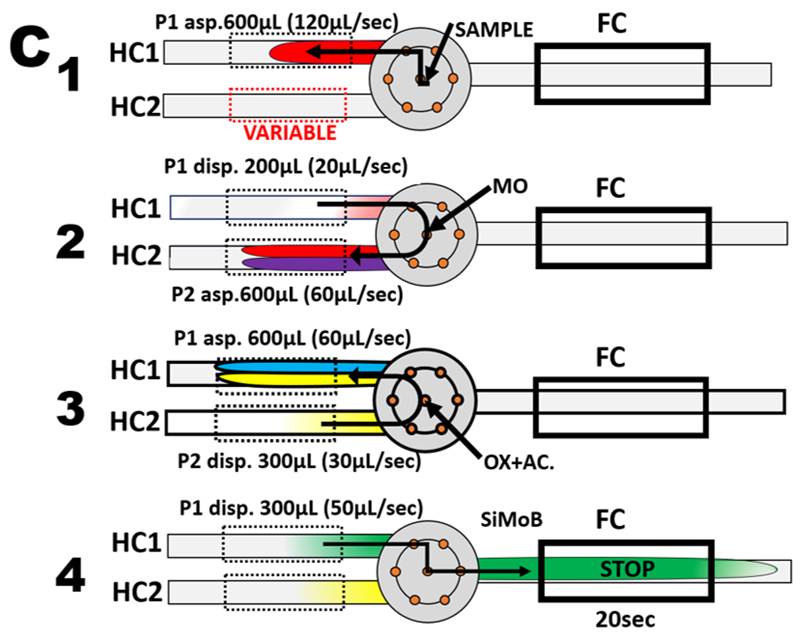
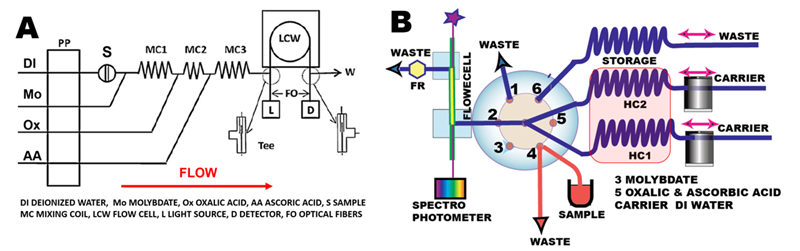 In programmable Flow Injection format (B), oxalic and ascorbic acids are blended and used in four steps, two reagent flow protocol (C) designed to operate in batch mode (Section 3.2.4 ). In the first step sample is metered by flow reversal into holding coil #1 (HC1). In the second step sample is transferred from HC1 to HC2 while molybdate is added at confluence point. In the third step SiMo and PMo, formed in HC2 are transferred back to HC1 while oxalate/ascorbic acids reagent is added at confluence point. Finally, the reaction mixture is transferred into the flow cell, where it is held for 20 seconds for absorbance monitoring at 810 nm.
In programmable Flow Injection format (B), oxalic and ascorbic acids are blended and used in four steps, two reagent flow protocol (C) designed to operate in batch mode (Section 3.2.4 ). In the first step sample is metered by flow reversal into holding coil #1 (HC1). In the second step sample is transferred from HC1 to HC2 while molybdate is added at confluence point. In the third step SiMo and PMo, formed in HC2 are transferred back to HC1 while oxalate/ascorbic acids reagent is added at confluence point. Finally, the reaction mixture is transferred into the flow cell, where it is held for 20 seconds for absorbance monitoring at 810 nm.
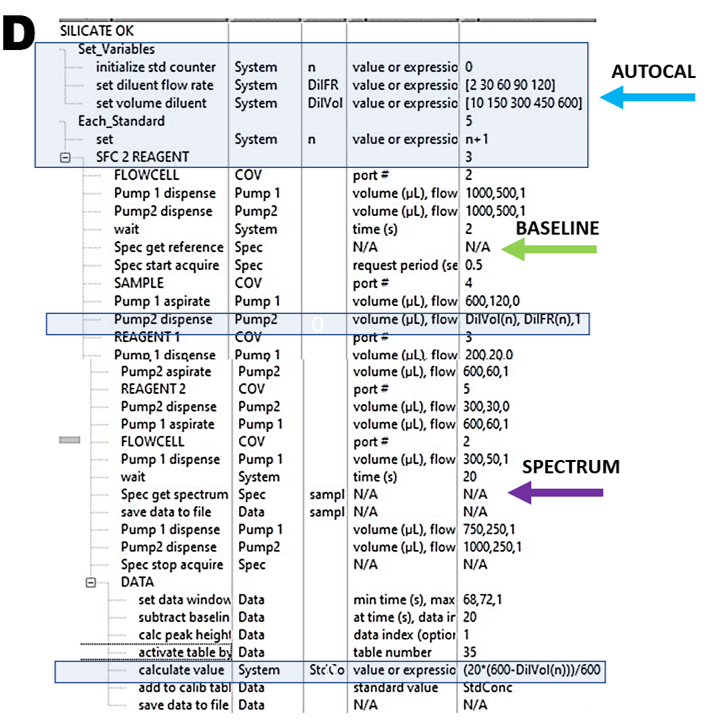
 Autocalibration by Single Standard Solution (ASSS) described in detail in Section 3.2.5. is based on stepwise dilution of Standard Solution by DI water carrier, while SS is being aspirated into HC1 during the first step of flow protocol (C). By aspirating always, the same volume (600 μL) into HC1 while increasing volume of diluent in five steps by means of software protocol (D) a five point calibration run and corresponding calibration graph are automatically generated.
Autocalibration by Single Standard Solution (ASSS) described in detail in Section 3.2.5. is based on stepwise dilution of Standard Solution by DI water carrier, while SS is being aspirated into HC1 during the first step of flow protocol (C). By aspirating always, the same volume (600 μL) into HC1 while increasing volume of diluent in five steps by means of software protocol (D) a five point calibration run and corresponding calibration graph are automatically generated.
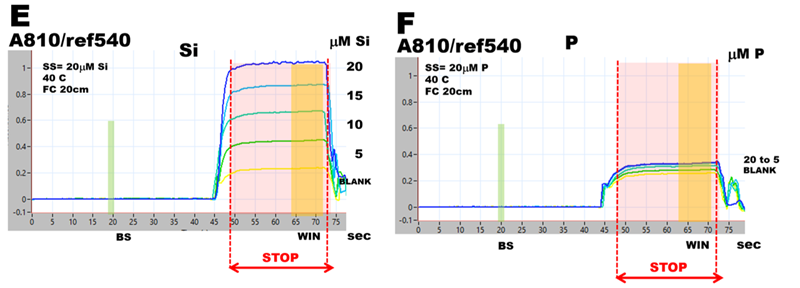
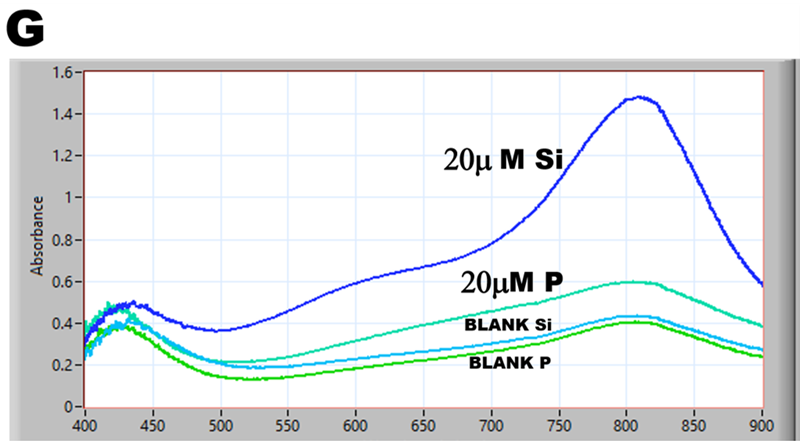
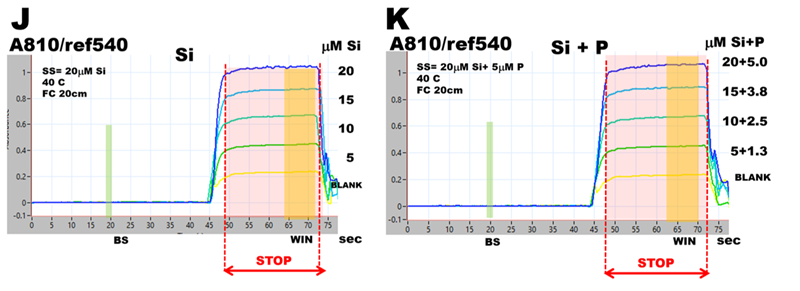
Phosphate interference in silica determination was investigated by using pFI manifold (B), software protocol (D), and reagents listed at the end of this chapter. Two ASSS calibration experiments were obtained, one by using 20 μM Si standard solution (E), the other by using 20 μM P standard solution (F), both standards prepared in DI water. Visual observation confirms that at this Si:P ratio 1:1 phosphate will significantly increase the concentration of silica determined at these conditions. To find out why, spectra of 20 μM Si, 20 μM P as well as blank (DI water) were recorded during WIN stop flow period and superimposed in fig (G). As one would expect, silica generates SiMoB spectrum showing maximum at 810nm and shape described in the literature. However, the spectrum obtained with 20 μM P has no maximum at 880nm – a feature typical for PMoB, while it exhibits maximum at 810nm and is similar in all respect to spectrum of SiMoB thus causing a significant interference. Equally unexpected are BLANK spectra obtained at Si and P runs when DI water is the sole analyzed component. The ‘black box’ of MoB chemistry is turning into box of Pandora.
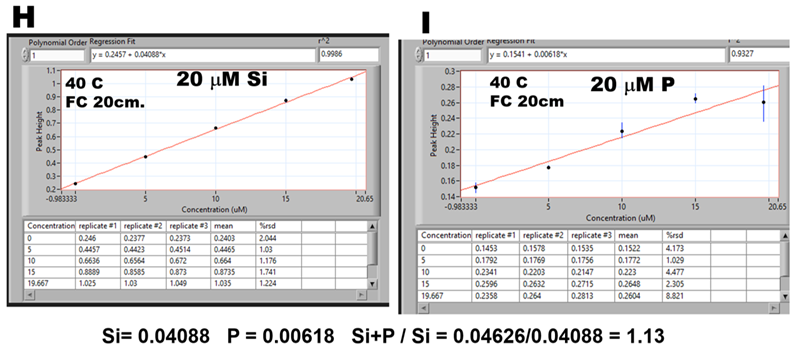
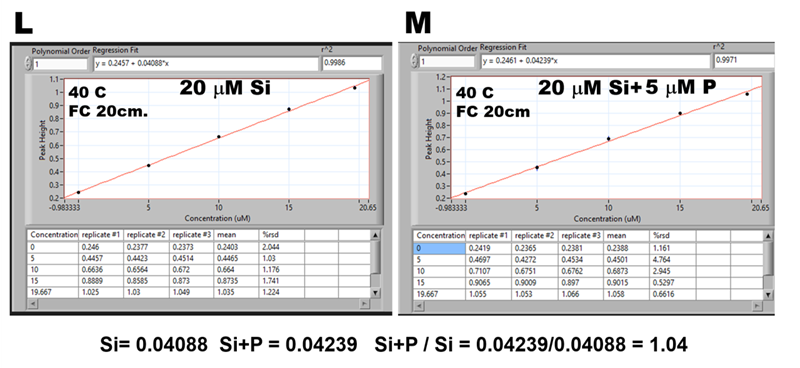
Let us quantify the P interference by using ratio of calibration lines obtained with 20 μM Si (H) and 20 μM P (I) standards. Since both calibrations are linear, and assuming that formation of measured MoB species is mutually independent, it follows that for any solution containing Si:P=1:1 the found Si value will be elevated by 13% throughout any concentration range. If this assumption is correct, then e.g. for Si:P = 4:1 the found Si value will be elevated by 3.25%, an error that is within the range of uncertainty of the method. To verify this assumption, 30 mL of 20 μM Si standard solution was spiked with 150 μL of 1.00 mM P and both Si (J) and Si+P (K) solutions were analyzed, and corresponding calibrations (L) and (M) were compared. The Si found value was elevated by 4% instead of 3.25% and predicted slope was only 0.04 mAU steeper than the found one.
In open ocean waters the Si:P ratios vary from 10:1 to up to 30:1 along the increasing depth profile, while Si concentration increases in the range of 0 to 180 μM and therefore the Si concentrations found by this method will not be affected by phosphate present. But use of this method for analysis of coastal and river water where Si:P ratio is lower should be critically examined.
DI water-based calibration for analysis of sea water is in detail discussed in Sections 3.2.4.2, 3.2.5.4., and [5]. Salinity affects reagent-based assays because it influences reaction rates of the chemical reactions involved. To mitigate this interference calibration standards are routinely prepared in sea water for sea water analysis. This approach has numerous drawbacks since SW must be collected in open ocean, purified from trace and biocomponents and stored. SW based standards can only be used for analysis of SW samples of the same salinity.
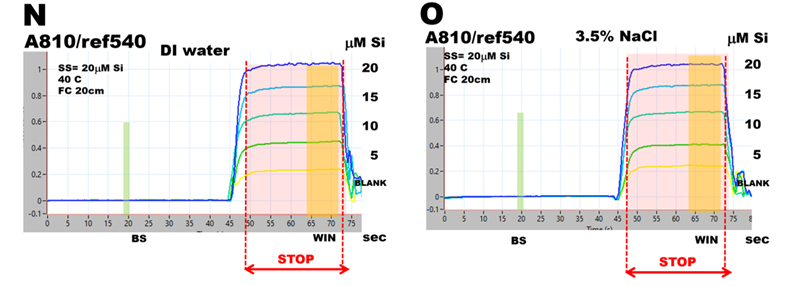
pFI batch technique performs absorbance measurement on reaction mixture that reached chemical equilibrium and is held stationary in a flow cell (Section 3.2.4. and [5]. Therefore, it is possible to design a flow protocol that will eliminate or minimize the influence of salinity and use DI water-based calibration for assay of SW samples. To evaluate how much the influence of salinity is mitigated by pFI-batch protocol, Single Standard Autocalibration is used to analyze 20 μM Si standard prepared in DI water and 20 μM Si standard prepared 3.5% NaCl DI water solution. (Salinity of 3.5% NaCl simulates salinity of open ocean water sea water -as shown in Section 3.2.5.4.)
Autocalibration run for DI water-based SS (N) was obtained in a way described above. The simulated SW calibration run (O) was obtained with SS prepared in NaCl solution. In order to maintain salinity of this standard during autocalibration, the DI water carrier delivered by pump 2 (P2) was replaced by 3.5% NaCl solution. The DI (P) and simulated SW (R) calibration graphs are identical since the ratio of slopes of calibration lines and calibration line offsets are close within 3%, and therefore DI calibration is suitable for analysis of SW samples.
.png.aspx?width=800&height=341)
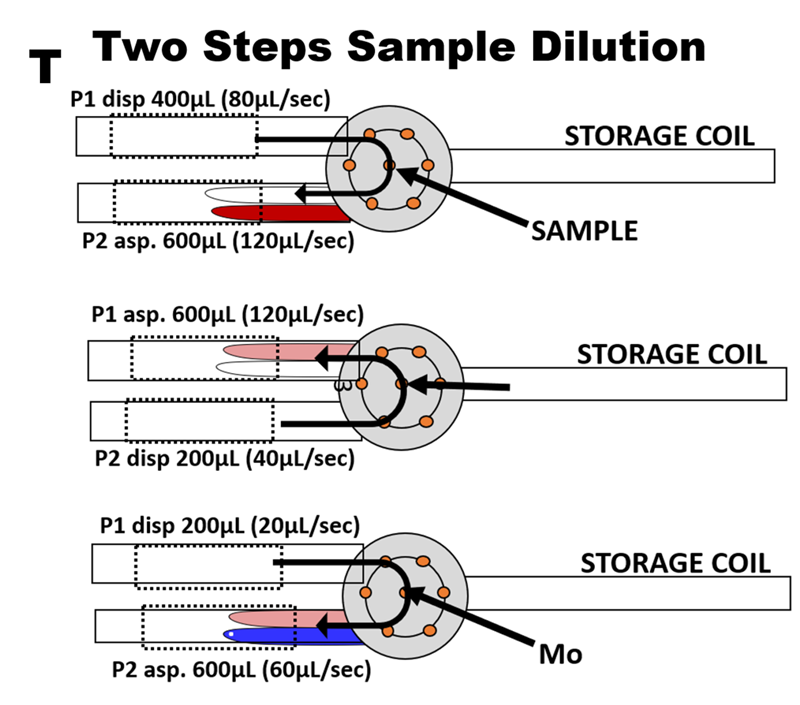
Extending calibration range by autodilution is necessary because concentration of silicate increases with increasing from 1 up to 200 μM Si. Because change of salinity does not affect DI based calibration, SW samples can be automatically diluted by DI water carrier supplied by pump 2 (P2). In this way Single Step Autodilution will extend 0 to 20 μM range up to 60 μM (S). The Two Step Autodilution (T), where DI water diluent is supplied by P1 and aspirated from holding coil will extend the range to 180 μM Si.
.png.aspx?width=800&height=490)
 Conclusion. Selectivity, or lack of it, can be efficiently evaluated by SS Auto calibration not only for target/interferant ratio but also for range of their concentrations and will serve as a tool for optimization of reagent-based assays.
Conclusion. Selectivity, or lack of it, can be efficiently evaluated by SS Auto calibration not only for target/interferant ratio but also for range of their concentrations and will serve as a tool for optimization of reagent-based assays.
The pFI based two-reagent method, presented here for the first time is not only feasible and highly reproducible, but also potentially useful for analysis of open ocean sea water and allows use of DI based calibration.
However, it is rather problematic to rely on method of which the underlying chemistry is unknown. While unbiased observation of spectra shown in fig. (G) suggests that all of them are of the same compound, present at different concentration, formation such compound in DI water (blank), or triggered by silicate or phosphate in proportion to their concentrations does not fit the presently accepted concept of chemistry of MoB related species [1]. The consequence of this finding is that use of oxalic acid to mitigate P interference on Si determination should be further investigated because kinetics and mechanism of decomposition of PMoB and SiMoB by oxalic acid proposed long time ago [6 and 1] are not yet well understood.
Composition of reagents
Silicate stock solution, containing 1000 microM Si was prepared weekly by diluting a commercial 1000ppm Si standard (15747-100ML, Fluka) with 0.5 N HCl. This stock solution was further diluted daily, to obtain the single standard containing 20.0 µM Si by DI water.. The silica solution had to be neutralized by HCl, because the silica standard is prepared in sodium hydroxide solution.
The mixed oxalic and ascorbic acid solution was prepared by dissolving 2g of oxalic acid anhydrous, 2g of ascorbic acid and 2g of SDS surfactant (CAS151-21-3, Thermo Scientific), in 100mL of DI water. The solution, prepared ½ hour before use to stabilize its reducing strength was stable for a week when stored in darkness at a room temperature
The acidified molybdate reagent was prepared by dissolving 1.0 g of ammonium molybdate tetrahydrate crystalline (A674-500, CAS12054-85-2, certified ACS, Fisher Scientific) in 50 mL of DI water. Next, 0.5 mL of conc. sulfuric acid (A300-500, CAS7664-93-9, certified ACS, Fisher Scientific) was added and then DI water was added to make a final volume of 100 mL. This solution was stable for one month.
Phosphate solution was prepared by diluting a commercial PO4 standard (LC185701, LabChem) in DI water. The 20µM P single standard solution was prepared before use.
References
[1] E. A. Nagul, I.D. McKelvie, P. Worsfold, S. D. Kolev. “The molybdenum blue reaction for the determination of orthophosphate revisited: Opening the black box” Anal.Chim.Acta. 890, (2015), 60.
[2] Jian Ma, R.H. Byrne “Flow injection analysis of nanomolar silicate using long path absorbance spectroscopy” Talanta 88 (2012), 484.
[3] A. K. Pettersson, Bo Karlberg “Simultaneous determination of phosphate and silicate in brackish water” Anal. Chim. Acta 378 (1999), 1283.
[4] C. X. Galhardo, J. C. Masini “Spectrophotometric determination of phosphate and silicate by sequential injection using molybdenum blue chemistry" Anal. Chim. Acta. 417, 2, (2000), 191.
[5] Mariko Hatta, Jaromir (Jarda) Ruzicka, Christopher I. Measures, Madeline Davis “Programmable flow injection in batch mode: Determination of nutrients in sea water by using a single, salinity independent calibration line, obtained with standards prepared in distilled water “Talanta 232 (2021) 122354
[6] R. A. Chalmers, A.G. Sinclair “Analytical applications of beta- heteropolyacids” Anal. Chim. Acta 34 (1966), 412.