Group of pathology and pharmacology of cardiovascular and metabolic disorders
Our research group specializes in cardiometabolic diseases. Cardiometabolic disorders are a group of pathological conditions that include Endothelial dysfunction, Atherosclerosis, Ischemic Heart Disease, Arterial hypertension, Insulin resistance, Diabetes mellitus type 1 and type 2, and Non-Alcoholic Fatty Liver Disease (NAFLD). The percentage of patients who have one or more of these illnesses over their lives is increasing worldwide.
.png.aspx?width=700&height=489)
Our main focus is to study endothelial dysfunction, atherogenesis, and liver alterations both in vivo in animal (mice) models and in vitro in cell cultures. Our main goal is to investigate the role of tissue and soluble Endoglin and its corresponding signaling in endothelial dysfunction, atherogenesis, and liver alterations in vivo and in vitro, and under pharmacological treatment.
What is Endoglin, and why are we interested in it
Endoglin (CD105) is a 180 kDa transmembrane glycoprotein and a coreceptor of the Transforming Growth Factor β (TGFβ) superfamily. There are two forms of endoglin currently studied, namely tissue (membrane) endoglin (ENG) and soluble endoglin (sENG) circulating in the blood. ENG is expressed mainly by endothelial cells, vascular smooth muscle cells, fibroblasts, hepatic stellate cells, activated monocytes, and macrophages. sENG is the extracellular domain of membrane ENG, which is cleaved by a membrane-type metalloproteinase-14 (MMP-14) and 12 (MMP-12). sENG enters the bloodstream, where it can be detected.
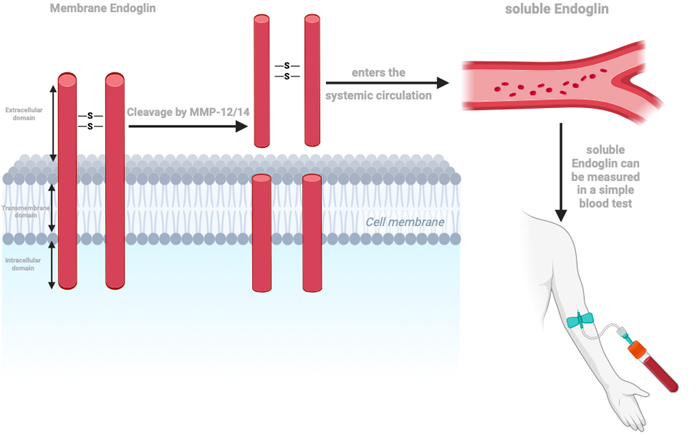
How is Endoglin related to our research?
We are currently investigating the role of ENG in cardiometabolic disorders.
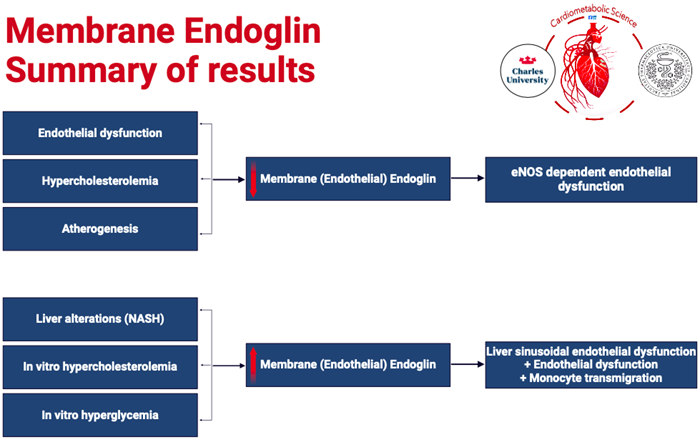
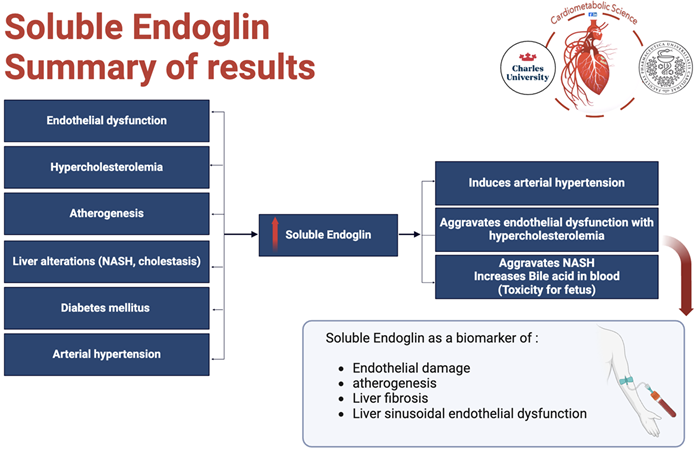
Endothelial dysfunction (ED) is a term that refers to impaired function of the lining of blood vessels (vascular endothelium), which could raise the risk of cardiovascular disease, such as atherosclerosis, high blood pressure, and thromboembolic events. ENG is expressed in the aorta by both endothelial cells and smooth muscle cells, it has been shown that ENG is linked to endothelial dysfunction with respect to its regulation of NO-dependent vasodilatation, as well as endothelial NO-synthase (eNOS) expression and activity. ENG activation may result in increased eNOS production, anti-inflammatory effects via inhibition of NF-κB signaling, activation of the transcription factor early growth response-1 (EGR-1), collagen production, and plaque stabilization, which might lead to an athero-protective mechanism in the vessel wall. Furthermore, sENG levels could be used as a biomarker in disorders linked with endothelial dysfunction and could indicate the efficacy of therapeutic interventions. Statins and LDL apheresis have been shown to reduce sENG levels in both mice and humans.
Non-alcoholic fatty liver disease (NAFLD), the hepatic manifestation of the metabolic syndrome, is characterized by fat accumulation and inflammation in the liver when there is no or little alcohol consumption. NAFLD patients may develop a more progressive form of the disease, called non-alcoholic steatohepatitis (NASH), which may progress to cirrhosis and permanent liver damage. NASH enhances liver ENG expression, resulting in higher amounts of sENG in plasma. Furthermore, the presence of additional high levels of sENG in the blood of transgenic mice led to an aggravation of pre-existing NASH by raising TAG and cholesterol accumulation in the liver and decreasing BA biliary secretion. Thus, sENG might be a circulating biomarker of NASH, making it easy to diagnose NASH in the early stages.
Cholestasis is a pathological condition in which bile formation or flow is impaired, resulting in fatigue, pruritus, and jaundice. Cholestatic liver injury, as well as NASH, have been associated with altered ENG expression, most notably in liver sinusoidal endothelial cells (LSECSs). It was also shown that cholestasis, like NASH, caused high sENG levels, cleaved probably by MMP-14 expressed predominantly in LSECs, implying that these cells are the predominant source of sENG in cholestasis and NASH pathologies, indicating sENG as a biomarker of liver injury.
All figures were created with BioRender.com.