In 2010, we found that ultrafast intramolecular charge transfer (ICT) proceeds at tetrapyrazinoporphyrazine (TPyzPz) extremely efficiently between a donor (typically amine or phenolate) and an acceptor (i.e. TPyzPz core). ICT is a competitive relaxation pathway to fluorescence emission and singlet oxygen production, but proceeds much faster. Thus, ICT quenches the excited states rapidly and no fluorescence and singlet oxygen procuction is observed. Focusing on this interesting phenomenon, we have described in detail structural factors influencing ICT efficiency. This knowledge was further used for the development of new class of promising red-emitting fluorescence sensors for both acidic and basic pH, CO2 and metal cations (see below), where the switching between OFF/ON states is based on enabling/blocking ICT to proceed.
The most important structural factors affecting ICT are as follows:
- ICT is more efficient at TPyzPz in comparison to Pc due to pronounced electron-deficient character of the macrocyclic core
.jpg.aspx?lang=en-GB&width=193&height=139) |
|
Vachova L, Novakova V, Kopecky K, Miletin M, Zimcik P, Effect of intramolecular charge transfer on fluorescence and singlet oxygen production of phthalocyanine analogues, Dalton Transactions, 2012; 41 (38): 11651-11656.
Novakova V, Reimerova P, Svec J, Suchan D, Miletin M, Rhoda HM, Nemykin VN, Zimcik P, Systematic investigation of phthalocyanines, naphthalocyanines, and their aza-analogues. Effect of the isosteric aza-replacement in the core, Dalton Transactions, 2015; 44: 13220-13233.
|
- Electron deficiency of TPyzPz core can be simply tunned by the attachment of suitable peripheral substituents. Selection of a proper substituent may be done using Hammett substituent constants (σp)
- Character of a donor (e.g. alkyl/aryl amine, etc.) and a substituent in close proximity to a donor plays an important role in ICT efficiency. Conjugation between a donor and acceptor must be maintained to enable ICT, because photo-induced electron transfer (PET), where the electron “jumps” through a space, cannot be used for sensing properties due to its significantly lower efficiency at TPyzPzs
.jpg.aspx?lang=en-GB&width=593&height=293) |
|
Novakova V, Hladík P, Filandrová T, Zajícová I, Krepsová V, Miletin M, Lenčo J, Zimcik P, Structural factors influencing the intramolecular charge transfer and photoinduced electron transfer in tetrapyrazinoporphyrazines, Physical Chemistry Chemical Physics, 2014; 16(11): 5440-5446. |
- Number of donors: One donor only is the best choice for senors because switching between OFF/ON states can be simply driven by surrounding environment. TPyzPzs with more than four donors remain still in OFF state and are used in different application – see quenchers of fluorescence.
Based on this knowledge, sensors for different fields has been described. The most important projects are as follows:
.jpg.aspx?lang=en-GB&width=247&height=247) |
 |
Sensors for acidic pH where the fluorescence between OFF/ON states increased ~80× (reaching fluorescence quantum yields in ON state ~0.30 in DMF) are described. Target TPyzPzs anchored into liposomes simulating a model of a biological membrane were sensitive to pH also in water. pKa values ranged between 2.2 and 4.2 making these compounds promising red-emitting sensors for more acidic pH range. |
Sensors for basic pH using phenol/phenolate donor pair to switch the sensors OFF/ON opened a new space not only to pH sensors but also for sensing gasseous CO2. A novel concept for fluorescence OFF/ON/OFF switching based on combination of deprotonation of central hydrogens in metal-free TPyzPzs and forming a phenolate upon addition of specific amounts of base was demonstrated for the first time. Sensing properteis have been described also in water using liposomes (pKa values ranged 12.5 - 12.7).
- Novakova V, Lochman L, Zajícová I, Kopecky K, Miletin M, Lang K, Kirakci K, Zimcik P, Azaphthalocyanines: Red Fluorescent probes for Cations, Chemistry - A European Journal, 2013; 19 (16): 5025-5028.
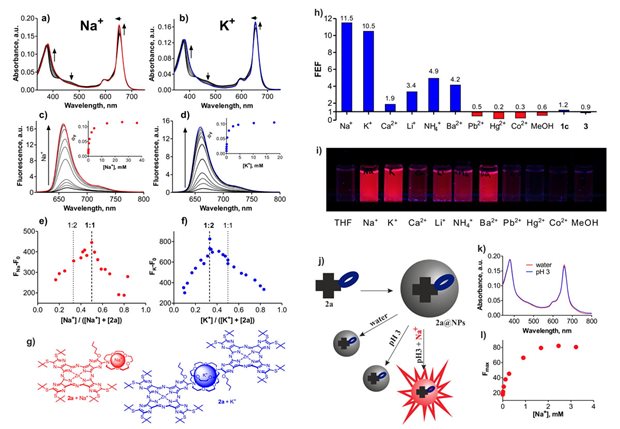
| Suitability of TPyzPzs to become effective sensors for metal cations is presented in this pilot project on TPyzPzs bearing 1-aza-15-crown-5 on periphery. Sensors were preferentially sensitive to K+ and Na+. Stoichiometry assessment disclosed different binding of Na+ and K+ into the aza-crown. Advantageously, sensors did not switched ON under acidic conditions due to suppressed basicity of donor moiety. Sillica nanoparticles have been found as the best delivery system enabling sensing of metal cations in water. |
|
 |
 |
This is a project focusing on the improvement of the selectivity to desired cations. The effect of size of aza-crown moiety was in detail studied on a series of TPyzPzs bearing 1-aza-12-crown-4, 1-aza-15-crown-5, 1-aza-18-crown-6 or 1-aza-21-crown-7 as a recognition moiety. Important relationships between aza-crown size and binding affinity were observed in the group of alkali metal cations, whereas were not noticed in the group of alkaline earth metal cations. Appropriate dissociation constants (KD) and fluorescence quantum yields in OFF/ON states have been determined in ACN and widely discused. |
- Lochman L, Svec J, Roh J, Kirakci K, Lang K., Zimcik P, Novakova V, Metal cation recognition in water by a tetrapyrazinoporphyrazine-based tweezer receptor, Chemistry - A European Journal, 2016, 22, 2417-2426.
| Rigidity of an aza-crown recognition moiety has been demonstrated as a key feature to substantially improve selectivity to desire metal cations. Interestingly, mono- or biphasic character of the binding isotherms was observed. This feature together with binding stoichiometry and magnitude of association constants (KA) indicated specific complexation of particular analytes. The lead compound was embedded into silica nanoparticles and advantageous sensing properties towards K+ were demonstrated in water (λF=671 nm, apparent KA=82M-1, increase of 17×), even in the presence of (supra)physiological concentrations of Na+ and Ca2+. |
|
.jpg.aspx?lang=en-GB&width=299&height=249) |